Ultrasensitive Detection of Hg2+ Ions With CVD Grown MoS2-Functionalized MgZnO/CdZnO HEMT
Heavy metals are naturally occurring in Earth's crust and have the property of accumulating in the ecosystem, posing health risks to humans, even in trace amounts. Mercury (Hg²⁺) is particularly toxic and can spread through natural and industrial sources, as well as bioaccumulate in the food chain.
Even small amounts of mercury can damage the brain, lungs, and kidneys if they build up in the body. Long-term exposure may lead to chronic illnesses or death, threatening both human health and ecosystems worldwide. Despite strict guidelines by the World Health Organization (WHO) for safe mercury levels in drinking water, reliable detection at ultralow concentrations remains a challenge.
Existing spectroscopy and electrochemical analysis methods, while sensitive, require expensive infrastructure, skilled operators, and are unsuitable for rapid, on-site testing. High-electron-mobility transistor (HEMT)-based biosensors for mercury detection are sensitive, fast, and stable, but gate-induced leakage can compromise their accuracy and sensitivity.
The researchers in this study developed a novel sensor employing a HEMT architecture enhanced with chemical vapor-deposited (CVD) molybdenum disulfide (MoS2), a material known for its unique two-dimensional structure and strong affinity for mercury ions.
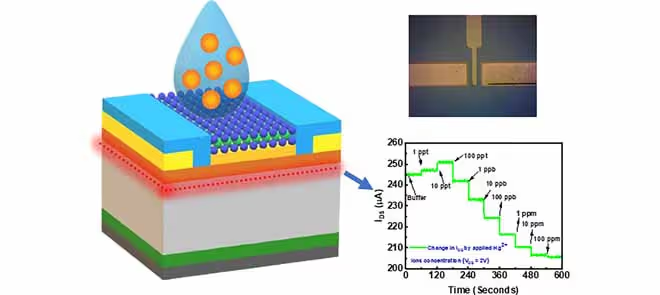
The proposed sensor utilizes a magnesium zinc oxide/cadmium zinc oxide (MgZnO/CdZnO) heterostructure, providing an electronically stable platform with high carrier mobility. MoS₂ was synthesized on a silicon dioxide/silicon (SiO₂/Si) substrate using CVD and transferred onto the transistor’s gate region to enable selective detection. The design eliminates the need for reference electrodes and minimizes leakage currents.
The sensing mechanism is rooted in the strong chemical interaction between mercury ions and sulphur atoms in MoS2. At low concentrations, mercury binds to sulphur, forming Hg-S complexes that initially increase the transistor’s current. At higher concentrations, additional mercury adsorption induces an electrostatic effect that reduces the current, allowing a clear distinction across a wide concentration range (1 ppt-100 ppm). These interactions are confirmed through spectroscopic analyses, validating mercury adsorption at the MoS2 surface.
The fabricated device demonstrates remarkable performance. It achieves an exceptionally low detection limit of 6.5 parts per trillion (ppt), which is well below international safety thresholds. The response time is under 4 seconds, ensuring near-instantaneous detection. The sensor also shows high selectivity, responding strongly to Hg2+ ions while remaining unaffected by other common heavy metals such as lead, copper, and cadmium.
The device also demonstrates repeatability and reproducibility, with consistent performance across multiple tests and devices. It can be regenerated by a simple heating step in buffer solution, achieving a recovery rate of over 99%. When benchmarked against existing mercury sensors, this MoS2-functionalized HEMT outperforms them in terms of detection limit, response time, and sensitivity (9.55 µA/ppb).
This work introduces a portable, ultrasensitive, and selective HEMT-based sensor for real-time mercury detection in water. With a detection limit of 6.5 ppt, sub-4-second response, and excellent reusability, it far surpasses existing technologies. Its compatibility with Internet of Things (IoT) integration and robustness make it ideal for large-scale environmental monitoring, ensuring safer water quality and improved public health protection.