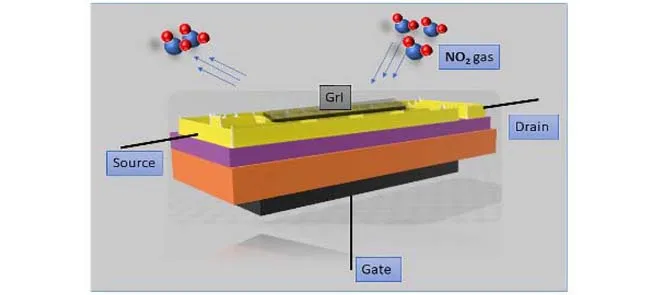
NO2 Gas Sensor Using Iodine Doped Graphene at Room Temperature with Electric Field Enhanced Recovery
Nitrogen Oxide (NO2), a major byproduct of different chemical industries, transports, combustion reactions, etc., is a serious environmental pollutant and can cause respiratory issues, eye irritation, and other health problems. Carbon nanomaterial-based FET gas sensors show many advantages, like low power consumption, miniaturization, low fabrication cost, fast response, and recovery time compared to conventional gas sensors. These are often used to detect Volatile Organic Compounds (VOCs), gas, pH, etc. For better sensitivity, Iodine (I2) was doped into multilayer graphene, which is used as the active layer of the FET gas sensor.
I-MLG is synthesized by exposing multilayer graphene (MLG) and Iodine (I2) to UV (ultraviolet) radiation in the presence of chloroform at room temperature. After the photolysis process, the exposed solution was filtered and washed with distilled water, and the final product I-MLG was obtained as a black color powder after drying the solution at 400C.
The Back gated FET was fabricated with Silicon (Si) as its substrate and SiO2 was grown on top of it by oxidation method. Using thermal evaporation, the gold electrode and inter digited electrodes (IDEs) were deposited for the back gate and source/drain structures. The gold coating on the Cu-IDEs prevents oxidation and increases the contact resistance. Sensor material (solution of I-MLG with ethanol) was deposited on the IDEs by the drop cast method. The sensor surface morphology of the nanostructures and elemental analysis were thoroughly compiled in the characterization study by Raman spectroscopy, SEM (Scanning electron microscopy), and EDX (Energy Dispersive X-ray analysis).
During the gas sensing, the gate voltage was kept at 0 V., so the sensing was not affected by the electric field. The decrease in sensor resistance due to the adsorption of NO2 molecules was registered using an LCR meter. To establish the selectivity of the sensor, the sensor was exposed to 50 ppm of other gases like N2, H2, NH3, CO, CO2, chloroform, ethanol, and 70% humidity one at a time. Except for humidity (13.7 %), the sensor shows less than 6 % sensitivity for other gases with an increase in resistance, whereas for 50 ppm NO2 it was -42.5% (decrease in resistance).
The response time of the sensor for 100 ppm of NO2 was 16 sec and has a self-recovery time of 1380 sec. after removal of the gas. This long recovery time can be explained by the formation of negative NO3̶ ions due to the interaction between NO2 gas and the sensor surface.
For fast recovery of the sensor, gate to source voltage VGS was varied -20 V. to +10 V. For positive voltages, the recovery process was unstable and showed high perturbation. But for negative biasing at the gate, there is an optimum value for VGS, which is found to be -15 V, with the shortest recovery time of 150 sec. Here the electric field produced by the biasing help the desorption process faster by repelling the negative NO3¯ ions from the sensor surface.
With the novel iodine-doped graphene as the sensing material, the gas sensor shows a reversible, rapid, and selective response towards NO2 sensing with an 88% decrease in recovery time owing to the electric field-assisted fast recovery.