WS2 Gas Sensor Based on Photothermocatalytic Effect for Ammonia Detection With High Response
Gas sensors based on metal oxides are widely used to detect various gasses. However, they require high operating temperatures to function effectively. To address the issue, researchers are exploring alternative materials like WS2, which promise better sensitivity and lower power consumption. This study focuses on developing a new type of gas sensor using minuscule WS2 sheets to detect ammonia, a crucial gas since it's hazardous.
The process of WS2-based gas sensing begins with synthesizing WS2 nanomaterials using a hydrothermal method. It involved combining sodium tungstate, hydroxylamine hydrochloride, and thiourea in water, adjusting the pH using either ammonia or hydrochloric acid, and then subjecting the mixture to heat in an autoclave. The resulting solid product was then washed and characterized using various techniques.
Scanning electron microscopy revealed the presence of lamellar structures, while X-ray diffraction confirmed the crystalline structure of WS2 with high purity. X-ray photoelectron spectroscopy provided insight into the elemental composition, highlighting the presence of sulfur and oxygen, which could influence the material's chemical interactions.
With the WS2 powder in hand, the researchers fabricated gas sensors by applying it to ceramic tubes equipped with heating elements. These sensors featured six electrodes, two for heating and four for gas detection, providing a platform for comprehensive gas sensing experiments.
The researchers embarked on a series of experiments to evaluate the performance of the WS2-based gas sensor. First and foremost was testing the sensor's selectivity against eight common gasses. Results demonstrated a high response to NH3 and low responses to other gasses like formaldehyde and xylene. This selectivity is crucial for ensuring accurate gas detection, as it prevents interference from non-target gasses.
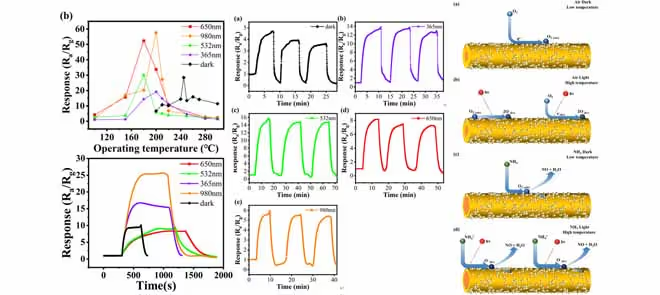
Continuing the exploration, the researchers delved into the optimal working temperature of the WS2 sensors under different lighting conditions. They discovered that light illumination effectively reduced the sensor's optimal operating temperature. In particular, infrared light produced a 57 times higher response at 200°C, while other wavelengths showed varying responses.
The impact of humidity on sensor performance was also investigated, revealing that higher humidity levels led to decreased response to NH3. This was due to water molecules occupying reactive sites on WS2's surface, thereby reducing the sensor's sensitivity to NH3.
Further experiments explored the sensor's response to different concentrations of NH3, demonstrating a proportional increase in response with higher NH3 concentrations. Additionally, the response-recovery times of the WS2 sensor were found to be shorter compared to others, likely due to accelerated reaction and recovery rates facilitated by photo-generated carriers absorbing energy from light.
The study also examined the influence of different ammonia concentrations under dark and light conditions. Light illumination significantly enhanced the sensor's response to NH3 compared to dark conditions, with specific wavelengths yielding higher response intensities. However, the sensor still required temperatures above room temperature to function and exhibited a delayed response to ammonia.
In conclusion, the study offers valuable insights into the sensitivity, selectivity, and optimal operating conditions of WS2-based gas sensors. By understanding these factors, researchers can work towards developing more efficient and reliable gas detection technologies for a wide range of applications, from environmental monitoring to industrial safety.