Susceptibility of Stimuli-Responsive Hydrogels With Embedded Magnetic Microparticles for Inductively Wireless Chemical Sensing
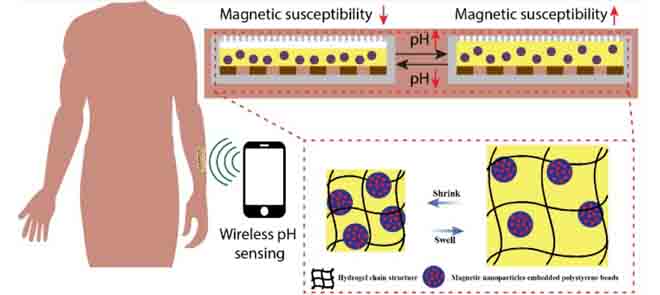
For sensing pH levels, hydrogels can be used in biomedical devices. Hydrogels are highly absorbent polymer networks that often use additive materials for enhanced responsiveness. For example, a pH sensitive hydrogel known as ferrogel is formed with magnetic microparticles in polystyrene (PS) beads. Ferrogel has broad utility in remote controllable drug release applications.
Ferrogels exhibit a reversible swelling and deswelling behaviour in response to the pH level. The sensitivity of pH measurement depends upon the magnetic susceptibility of the ferrogel-based sensor. The magnetic susceptibility of a material is its ability to get magnetized in an applied magnetic field. Therefore, for precise measurement of the pH levels, it is crucial to characterize the change in magnetic susceptibly of the ferrogel-based sensor with respect to the change in pH values.
For this, ferrogel PS beads are exposed to a magnetic field, which causes the magnetic nanoparticles to move and align to behave as tiny magnets. The degree of movement of the PS beads under magnetic force depends upon the surrounding environment. The pH level acts as a chemical stimulus to ferrogel and changes its volume resulting in swelling or shrinking. Due to the change in the volume of the bead, its magnetic susceptibility changes. Therefore, by backtracking, the chemical stimulus (pH level) is detected by measuring the magnetic susceptibility of the ferrogel.
Deformation in ferrogel in response to the pH level causes a change in rotation strength and concentration of PS beads inside the ferrogel. It leads to a change in the magnetic susceptibility of ferrogel. The size distribution of PS beads is quantitatively examined by experiments. For this, ferrogels are immersed in pH 4 and pH 6, respectively, and allowed to reach their swelling/shrinking equilibrium. After that, PS beads distribution is measured using a particle size analyzer, and magnetic susceptibility is analyzed using a superconducting quantum interference device.
Measurement results show that ferrogel swelling at a higher pH value results in less confinement of the beads, which eventually increases the susceptibility of the ferrogel. Measurements also showed that higher pH results in higher swelling and increased value of magnetic susceptibility. Therefore, magnetic energy stored in ferrogel also becomes a function of ferrogel swelling and hence on pH value. This helps detect any changes in the environment of the ferrogel by measuring the changes in the stored magnetic energy in the ferrogel.
The work of the researchers provides a better understanding of the chemo-mechano-magnetic kinetics of ferrogel. An invasive wireless chemical sensing approach can also be developed with the help of ferrogels. For this, a ferrogel film is created on top of a planar implantable inductor. pH-induced thickness variation of ferrogel film changes the inductance value, which can be sensed by measuring the self-resonating frequency of the inductor with an external antenna. The resonant frequency changes as the pH level changes. The same principle of wireless magnetic sensing can also be applied to detect other chemical stimuli such as glucose, temperature, and some specific ions.