Optimization of Lab-On-a-CD by Experimental Design and Machine Learning Models for Microfluidic Biosensor Application
With technological advancements, researchers are exploring strategies to optimize microfluidic systems for better patient care and develop efficient disease monitoring and diagnostics systems. The paper explores the optimization of Lab-On-a-CD (LACD) systems for microfluidic biosensors using experimental design and machine learning models to develop enhanced devices.
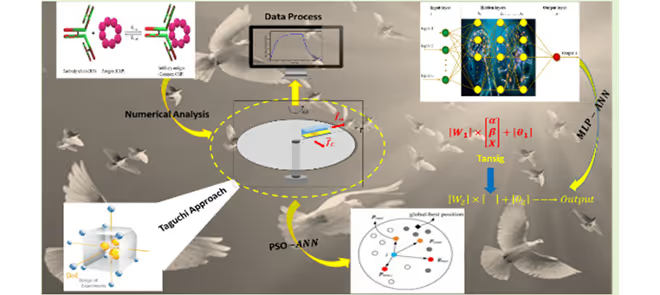
This study explores the intricate dynamics of microfluidic biosensors, with a focus on improving the accuracy of performance predictions using advanced computational models, specifically Artificial Neural Networks (ANN) and Particle Swarm Optimization-ANN (PSO-ANN). The velocity field deviation in radial and non-radial models where centrifugal forces significantly influence fluid distribution was analyzed. The parabolic velocity profile of the laminar flow affected by the Coriolis force is shifted towards the sidewall, with a remarkable deviation of 43.72% at a rotational speed of 100 rad/s.
Furthermore, the study investigates the binding kinetics of the complex reactive proteins (CRP) within the microfluidic biosensor, a critical aspect that determines the detection time of the analyte-ligand interaction. By analyzing 81 test scenarios with combinations of key parameters such as rotational speed, biosensor position, angular orientation, and radial displacement, the research identifies conditions that lead to the shortest and longest detection times. The results show that a radial model with specific settings outperforms a non-radial model in detection efficiency.
The ANN model is then evaluated for its ability to predict biosensor response times based on the above input variables. Optimizing the number of hidden layer neurons is critical to improving the accuracy of the model.
Through systematic experiments, the study determines that a 4:13:1 architecture (four inputs, thirteen hidden layer neurons, and one output) is optimal, as evident by the high regression coefficients and low mean squared error. It also compares the performance of the ANN model with the PSO-ANN model, highlighting the superior predictive accuracy of the latter.
The Taguchi method is used to optimize the PSO-ANN algorithm, focusing on parameters like cognitive and social coefficients, inertia weight, and particle size. By maximizing the signal-to-noise (S/N) ratio, the research identifies the best combination of these parameters, thus significantly reducing prediction error.
The PSO-ANN model configured with specific optimized parameters achieves an exceptionally high regression coefficient close to 1.00. The performance metrics, including RMSE (Root Mean Square Error), VAF (Variance Accounted For), and MAPE (Mean Absolute Percentage Error), consistently show that the PSO-ANN model outperforms the standard ANN model in terms of prediction accuracy. At the 1000th iteration, the cost value for the PSO-ANN model was impressively low, indicating its effectiveness in minimizing error and improving prediction reliability.
The paper presents a comparative analysis of the ANN and PSO-ANN models in predicting the detection time of rotating microfluidic biosensors. The results show that while both models are statistically significant, the PSO-ANN model provides superior performance as evident by higher correlation coefficients and lower prediction errors. This research highlights the potential of PSO-ANN models to set new standards in biosensor technology, providing more accurate and reliable predictions that could significantly lead to advancement in the field and offers promising insights for the future of biosensor technology.