Dual Electronic and Optical Monitoring of Biointerfaces by a Grating-Structured Coplanar-Gated Field-Effect Transistor
The study of interactions at the solid-liquid interface is crucial for understanding biological processes and developing advanced biosensors. Traditional biosensors often rely on single-mode detection, such as surface plasmon resonance (SPR), which detects mass changes near a metallic surface, or electrolyte-gated field-effect transistors (FETs), which are highly sensitive to surface charge variations.
However, biomolecular interactions are inherently multiphysics in nature—simultaneously affecting mass, charge, and dielectric properties—making single-mode sensing insufficient. Over time, multimode sensing platforms have evolved to integrate optical and electronic transduction in a single device, enabling simultaneous detection of mass and charge changes. For instance, integrating SPR with FETs enables label-free, multiparametric analysis, providing deeper insights into complex biomolecular interactions.
Despite their promise, these approaches face challenges like bulky components, complex gate designs, and limited optical probing. These challenges have driven interest in miniaturized SPR alternatives—such as metallic nanostructures and nanohole arrays—that offer more scalable, versatile sensing solutions.
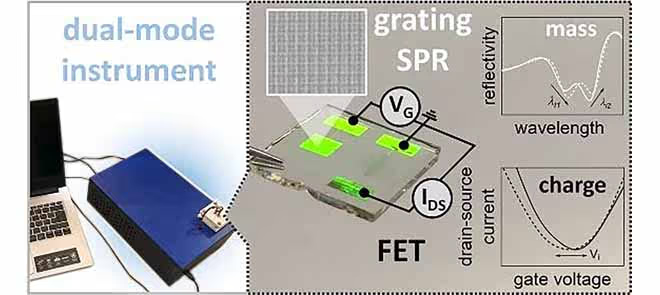
The study introduces a new multimodal sensor system, offering the ability to perform simultaneous optical and electronic readout directly on its gate surface. This innovative system combines grating-coupled surface plasmon resonance (SPR) for optical detection with a coplanar-gated field-effect transistor (FET) architecture, integrating both sensing capabilities onto a single chip.
The sensor leverages reduced graphene oxide (rGO) as the semiconductor material for the FET's electronic readout, while a metallic gate electrode, intricately corrugated with a multi-diffractive structure, facilitates optical probing with resonantly excited surface plasmons. The nanostructured architecture of the sensor is designed to support both sensing modalities without compromising the sensitivity or performance of either, offering a compact, efficient solution for advanced bioanalytical applications.
The utility of this dual-mode sensing system was demonstrated through an application involving a poly-L-lysine (PLL)-based linker layer, functionalized with "clickable" dibenzocyclooctyne (DBCO) groups for covalent binding of azide-modified biomolecules like deoxyribonucleic acid (DNA) oligomers. Using simultaneous electronic and optical measurements, the study revealed subtle yet critical insights into how chemical functionalization affects biointerface behaviour. As the density of DBCO attached to their backbone increased, the PLL chains exhibited folding and internalization of DBCO moieties, consequently reducing the coupling yield for DNA oligomers.
These results highlight the unique advantage of simultaneous mass and charge sensing, which can uncover subtle interfacial phenomena that would be overlooked using conventional single-mode approaches. Furthermore, tuning the multiperiodic grating periods allowed precise control over the operating wavelength, offering application-specific flexibility that is difficult to achieve with other configurations.
Relying solely on industry-compatible nano- and microfabrication techniques, the proposed sensor system supports scalable, low-cost production of disposable sensor chips without complex lithography. Paired with compact instrumentation, the portable, user-friendly system enables real-time detection of mass and charge, supporting multiparametric analysis. This advances the study of biomolecular interactions at solid–liquid interfaces and guides future biosensor development using organic electronics.