A Wireless, Passive Carbon Nanotube-Based Gas Sensor
The carbon nanotube (CNT)’s unique structure and material properties have put it at the forefront of practical applications such as in field emission devices, electronic switches, random access memory, actuators, gas sensors etc. Their excellent sensitivity and fast response time have paced the advancement of multiwall carbon nanotube (MWNT) based gas sensors. Gas sensors have attracted attention for their environmental monitoring, space exploration, industrial and commercial applications.
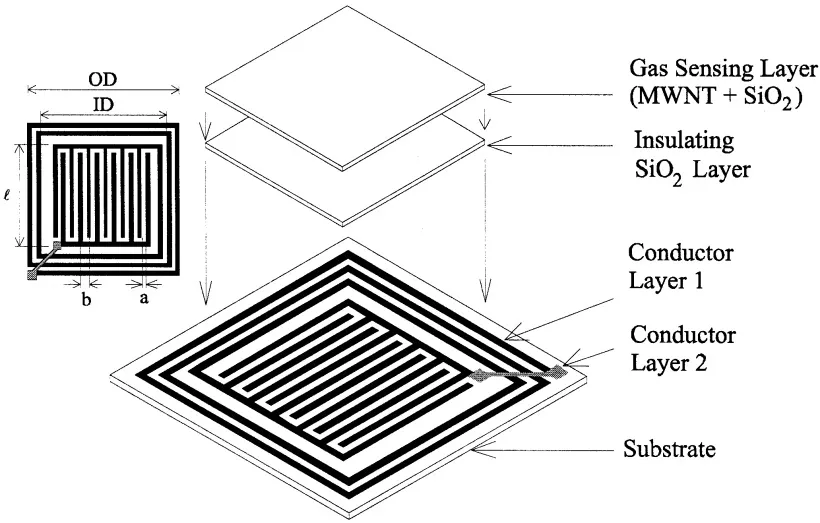
A MWNT gas sensor is made of composite layers. A planar inductor interdigital capacitor (LC) pair is printed on a copper clad substrate. It is then coated with an electrically insulated SiO2 layer followed by MWNT-SiO2 composite layer. SiO2 acts like a medium that binds the MWNTs to the sensor to make it gas-sensitive.
When the sensor is exposed to gases like carbon dioxide, oxygen, and ammonia, the relative permittivity and the conductivity of the adjacent MVNT-SiO2 composite change, consequently changing the sensor's resonant frequency. The sensor resonant frequency can be remotely tracked and monitored with a loop antenna in varying humidity, temperature conditions. The sensor response is found linear within 0-60% RH and 180C-430C temperature. The sensor response time is approximately 45 seconds for CO2, 4 minutes for O2 and 2 minutes for NH3. Based on the measured changes in MWNT permittivity and conductivity on exposure to gases, the concentration of CO2, O2, and NH3, can be determined.
For gas sensing, the sensor is placed in a sealed chamber in which gas concentration was controlled with a mass flow controller. A loop-antenna is placed remotely, and changes in permittivity and conductivity are measured by monitoring changes in resonant frequency. On altering the concentration of CO2 in the chamber, the permittivity and conductivity both decreases. Whereas, the presence of O2 decreases permittivity but increases conductivity of the sensing material. This is obvious too, as CO2 being a reducing agent, injects electrons in the p-type MWNT which combine with holes, hence conduction lowers. On the other hand, oxygen being an oxidizing agent extracts electrons from the p-type MWNT, which increases the conductivity. For CO2 and O2 detection, the sensor response is linear and reversible, and no hysteresis is observed.
The sensor’s response is found different in presence of NH3. The sensor shows both reversible and irreversible increase in the complex permittivity, which is caused by the difference in bonding mechanism between NH3 and MWNT. MWNTs absorbs ammonia molecules through two mechanisms: physisorption (weak bond) and chemisorption (strong bond). When NH3 concentration is reduced by adding dry air N2, the weak electrostatic bonds between the physisorbed NH3 and MWNTs are overcome by the kinetic energy of moving N2 that releases NH3, causing a reversible change. Conversely, the strong bonds of chemisorbed NH3 remain intact which builds up the density of NH3 on the MWNT surface causing irreversible changes. Both effects put together increase the permittivity. On the other hand, NH3 being a reducing agent like CO2 decreases the conductivity.
The measured changes in the complex permittivity are approximately twice as observed in CO2, indicating the MWNTs have a higher affinity for NH3. Whereas it is ten times smaller in presence of O2 than in case of CO2. All these results are reported at 0% RH and 230C temperature.
MWNT-SiO2 composite is a passive material for sensing. Hence, it could be wirelessly connected and no internal batteries are required to power the sensor. These characteristics make the gas sensor apt for remote long-duration wireless monitoring applications, making it possible to monitor even inside opaque, sealed containers.