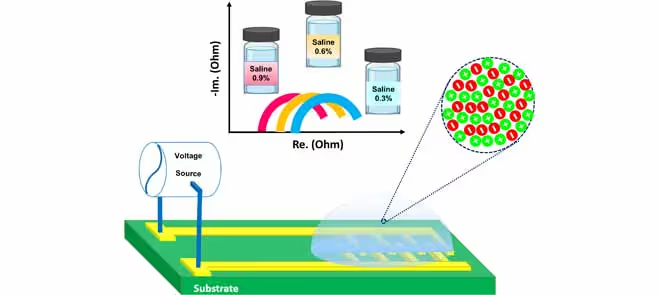
A Low-Cost Sensor for Assessment of Aqueous Solutions Properties
Interdigitated electrodes (IDEs) are popular in sensor design due to their easy fabrication, miniaturization, lab-on-chip integration, fast response, and suitability for small sample solutions. When combined with electrochemical impedance spectroscopy (EIS), IDEs form a robust and convenient analytical platform with acceptable accuracy. While standard cleanroom techniques are commonly used for sensor fabrication, they are costly and often inaccessible to smaller labs and startups. Printed circuit board (PCB) technology offers a cost-effective alternative for producing IDE-EIS sensors.
This paper reports on designing, developing, testing, and characterizing a novel, affordable IDE-EIS sensor fabricated on a PCB platform. The sensing structures in this work were fabricated using an FR4 substrate, with the electrode surfaces finished using electroless nickel immersion gold (ENIG). Two versions of the proposed sensors were developed, identical in all physical and geometric aspects except for the electrode finger width (250 µm/500 µm). EIS measurements were first carried out in the “dry” mode (with no electrolyte) and then used for pH and concentration measurements in the “wet” mode to characterize the sensors. The tests were conducted at room temperature (27°C) and across a frequency range from 10 Hz to 50 kHz.
The sensors were initially characterized in the dry mode using a general equivalent electrical circuit, which was subsequently modified to best fit the collected data. The circuit model parameters were determined by fitting the model to the data acquired in the dry mode.
In the wet mode, two series of tests were conducted to assess sensitivity to concentration and pH levels. For concentration sensitivity, saline solutions with concentrations ranging from 0.03% to 0.9% were used, while phosphate buffer solutions covering a pH range of 4 to 7.6 were used for pH sensitivity testing.
Experimental results indicated that the series resistance of the sensor’s electrochemical cell decreased as the solution concentration increased, which served as the basis for sensor calibration. Corresponding calibration curve equations were then derived. The findings also demonstrated that the developed sensors could reliably differentiate between samples differing by as little as 0.03% in concentration.
The RRC, RRCW, and RRQ electrical circuit models were fitted to the EIS data from pH tests. Based on the achieved results, two circuit parameters were identified as suitable for sensor calibration: (i) the resistance R1 in the RRC model showed an almost linear response with an absolute sensitivity of 91.802 Ω/pH across the entire pH range, with relative sensitivity varying from 56.35% at low pH to 70.36% at high pH; and (ii) the Q parameter in the RRQ model exhibited nonlinear response with relative sensitivity ranging from 248.4% at low pH to 471.96% at high pH.
In conclusion, the proposed EIS-based IDE sensor fabricated on a PCB offers an affordable and dependable tool for analyzing aqueous solution properties such as electrolyte concentration and pH. The sensor’s potential extends to applications such as disease diagnosis, biological research, food production, environmental temperature monitoring, and food and beverage quality assurance while facilitating deeper investigation into electrochemical system physics.